By Elizabeth King
Collaborators: Dr. Laura Bilenker, Anaïs Fourny, Genna Patton, Evelyn Freres, Dr. Dominique Weis, Dr. Ye Zhao
Iron (Fe) is of great interest to earth scientists and isotope geochemists because of its importance in the biogeochemical cycling of energy and nutrients, and its various applications in reconstructing magmatic and paleo-climatic processes (e.g., Rouxel et al., 2005; de Jong et al., 2007; Heimann et al., 2008).
High-precision Fe isotope analyses were made possible with the advent of multi-collector inductively coupled plasma mass spectrometry (MC-ICP-MS) within the last two decades. However, still remaining are several processes associated with MC-ICP-MS, causing instrumental mass fractionation (e.g., sample uptake, ionization, and interferences), that must be investigated in order to continue advancing Fe isotope measurements.
One of my research projects, in collaboration with Dr. Laura Bilenker, focuses on determining the direction and magnitude of matrix effects on Fe isotope measurements. Matrix effects occur when the mass load of Fe in a sample is different from that of a standard, leading to non-linear mass bias within the instrument. These matrix effects can be caused by the element of interest (self-induced), or by other elements.
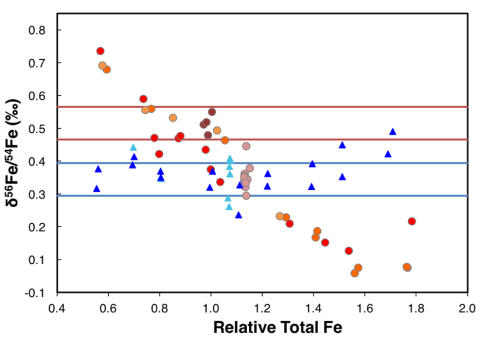
The goal of this Fe isotope study was two-fold: 1) to confirm a baseline for the self-induced matrix effect by using clean standard solutions and 2) to investigate and quantify the effect of additional elements from geological materials on the ratio offset caused by concentration mismatching. MAGNET Trainees Anaïs Fourny, Genna Patton, and Evelyn Freres show that the matrix effects occur when samples are run in dry plasma (Fig. 1).
Dr. Laura Bilenker and I designed two experiments to determine the direction and magnitude of self-induced and matrix effects on Fe isotope measurements. We performed experiments using pure Fe standard solutions to determine whether self-induced matrix effects were observable during Fe isotope analyses over a wide range of mismatched sample and standard concentrations.
Iron isotope ratios were measured using a Plasma 3 MC-ICP-MS in pseudo-high resolution at the Nu Instruments headquarters in Wrexham, Wales, UK (Figs. 2 and 3). The experiments were continued at PCIGR on a Nu Plasma 1700 (true high resolution) and Nu Plasma II (pseudo-high resolution).
Qualitatively, our results were consistent amongst the three instruments whereby when “sample” concentrations matched that of the bracketing standard, we observed no isotopic offset, but when the two solutions differed in concentration, we observed a measurable isotopic offset.
In addition, we measured a suite of reference materials over a range of mismatched sample and standard concentrations to test whether other elements that may be in a natural solution could cause matrix effects. The composition of these reference materials varied from ultramafic to felsic igneous rocks, Fe- and Mn- nodules, and soil.
Despite being widely observed, little work has been done to quantify the effect of matrix on Fe isotope analyses. These results will help to form a comprehensive dataset to assess reproducibility and potential instrument-specific influences. Ultimately, these results will be used to design a matrix-based correction for ratio offsets caused by mismatched standard-sample concentrations.
References
de Jong, J., Schoemann, V., Tison, J.-L., Becquevort, S., Masson, F., Lannuzel, D., Petit, J., Chou, L., Weis, D., Mattielli, N., 2007. Precise measurement of Fe isotopes in marine samples by multi-collector inductively coupled plasma mass spectrometry (MC-ICP-MS). Anal. Chim. Acta, 589: 105–19.
Heimann, A., Beard, B.L., Johnson, C.M., 2008. The role of volatile exsolution and sub-solidus fluid/rock interactions in producing high 56Fe/54Fe ratios in siliceous igneous rocks. Geochim. Cosmochim. Acta, 72: 4379–4396.
Rouxel, O.J., Bekker, A., Edwards, K.J., 2005. Iron Isotope Constraints on the Archean and Paleoproterozoic Ocean Redox State. Science, 307(5712): 1088–1091.

